- The pressure exerted by vapours over the liquid surface at equilibrium is called vapour pressure of the liquid.
- If solute is non volatile solid or liquid the vapour pressure of solution is equal to partial vapour pressure of solvent in the solution.
- If the solute is volatile solid or liquid, then vapour pressure will be equal to the sum of partial vapour pressure of solute and that of solvent.
Raoult’s Law:
“The partial vapour pressure of any component in the solution is directly proportional to its mole fraction”.
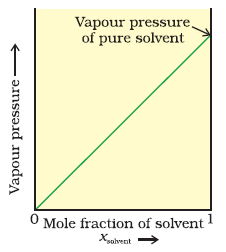
For a binary solution of two components A and B,
PA = XA
PB = XB
Where
P0A = vapour pressure of component A in pure state.
PA = vapour pressure of component A in the solution.
P0B = vapour pressure of component B in pure state.
PB = vapour pressure of component B in the solution
Limitations of Raoult’s Law
- Raoult’s law is applicable only to very dilute solutions.
- It is applicable to solutions containing non-volatile solute only.
- It is not applicable to solutes which dissociate or associate in a particular solution
Raoult’s Law in Combination with Dalton’s Law of Partial Pressure:
PT = XA P0A + XB P0B = P0B + (P0A -P0B) XA
Where
PT = Total Vapour Pressure of the Solution.
Ideal and Non-Ideal Solutions:
- Ideal Solution:
?These solutions obey Raoult’s law at all compositions of solute in solvent at all temperature
Two liquids A and B form and ideal solution when A –A and B–B molecular attractions will be same and hence A–B molecular attraction will be almost same as A–A and B–B molecular attraction.
For Ideal Solution:
1. dHmixing = 0, i.e. no heat should be absorbed or evolved during mixing
2. dVmixing = 0, i.e. no expansion or contraction on mixing
Examples , Ethyl chloride and ethyl bromide, n–hexane and n–heptane , CCl4and SiCl4
- Non-Ideal Solution:
?These solutions deviate from ideal behaviour and do not obey Raoult’s law over entire range of composition.
For non ideal solutions,
1. dHmixing ≠ 0
2. dHmixing ≠ 0
Here we may have two cases
A) Positive Deviation:
1. PA > XA & PB > XB
2. dHmix > 0
3. dVmix > 0
Example: Cyclohexane and Ethanol
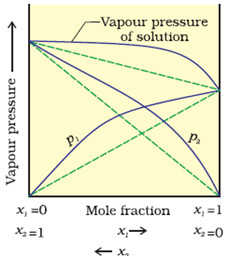
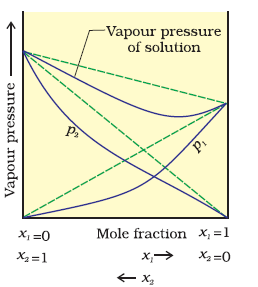
B) Negative Deviation:
1. PA > XA & PB > XB
2. dHmix < 0
3. dVmix < 0
Colligative Properties
The properties of dilute solutions which depend only on number particles of solute present in the solution and not on their identity are called colligative properties (denoting depending upon collection).
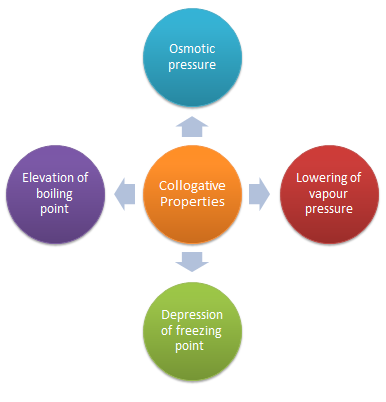
Lowering of Vapour Pressure by a Non-Volatile Solute
The relative lowering of vapour pressure of a solution containing a non-volatile solute is equal to the mole fraction of the solute present in the solution.
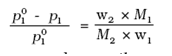
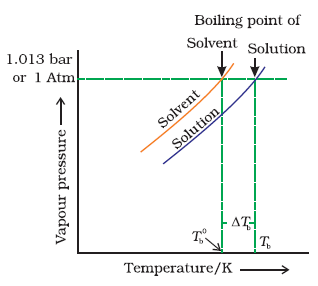
Elevation of Boiling Point by a Non-Volatile Solute :
Since the addition of a non-volatile solute lowers the vapour pressure of the solvent, the vapour pressure of a solution is always lower than that of the pure solvent, and hence it must be heated to a higher temperature to make its vapour pressure equal to atmospheric pressure.
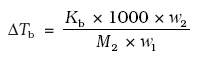
where M1 = molecular weight of solute and w2 and w1 are weights of solute and solvent
Depression of Freezing Point by a Non-Volatile Solute:
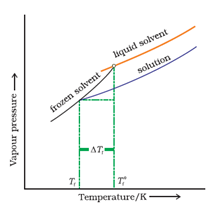
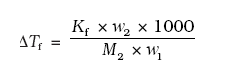
where M1 = molecular weight of solute and w2 and w1 are weights of solute and solvent
Osmosis and Osmotic Pressure:
- Osmosis: The phenomenon of the passage of pure solvent from a region of lower concentration (of the solution) to a region of its higher concentration through a semi-permeable membrane.
- Osmotic Pressure: Excess pressure which must be applied to a solution in order to prevent flow of solvent into the solution through the semi-permeable membrane.
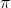 V = nRT
V = nRT
where
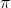 = Osmotic pressure
= Osmotic pressure
V = volume of solution
n = no. of moles of solute that is dissolved
R = Gas constant
T = Absolute temperature
Isotonic Solutions: A pair of solutions having same osomotic pressure is called isotonic solutions.
Abnormal Molecular Weight and Van't Hoff Factor:
Van't Hoff Factor:
Van't Hoff, in order to account for all abnormal cases introduced a factor i known as the Van't Hoff factor, such that

Degree of Association:
The fraction of the total number of molecules which combine to form bigger molecule
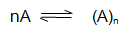
Let a be the degree of association, then,
The number of unassociated moles = 1-

The number of associated moles = /n
/n
 /n
/n
Total number of effective moles = 1- +
+ /n
/n
 +
+ /n
/n 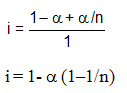
Obviously, i < 1
Degree of Dissociation
The fraction of the total number of molecules which dissociates in the solution, that is, breaks into simpler molecules or ions.
KCl ↔ K+ + Cl-
1-





Thus, the total number of moles after dissociation = 1- +
+ +
+ = 1+
= 1+
 +
+ +
+ = 1+
= 1+
Hence, i = (1+ )/1
)/1
 )/1
)/1
i = 1+ = 1+ (2–1)
= 1+ (2–1)
 = 1+ (2–1)
= 1+ (2–1)
In general, i = 1+ (n–1) ,
,
 ,
,
Where, n = number of particles ( ions) formed after dissociation
From the above formula, it is clear that i > 1
 All the three states of matter (gas, liquid or solid) may behave either as solvent or solute. Depending on the state of solute or solvent, mainly there may be the following seven types of binary solutions.
All the three states of matter (gas, liquid or solid) may behave either as solvent or solute. Depending on the state of solute or solvent, mainly there may be the following seven types of binary solutions.
0 comments:
Post a Comment