- Rate of change of extent of reaction is the rate of reaction.
- Rate of reaction is positive for product and negative for reactant.
- For reaction aA →bB Rate =1/b(Δ[B]/ Δ t) = -1/a (Δ [A]/ Δt)
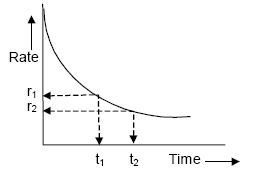
- It goes on decreasing as the reaction progress due to decrease in the concentration(s) of the reactant(s).
- Unit of rate of reaction : mol L-1 s-1
- The rate measured over a long time interval is called average rate and the rate measured for an infinitesimally small time interval is called instantaneous rate.
- In a chemical change, reactants and products are involved. As the chemical reaction proceeds, the concentration of the reactants decreases, i.e., products are produced.
- The rate of reaction (average rate) is defined as the change of concentration of any one of its reactants (or products) per unit time.
Order of Reaction

|
For reaction aA + bB + ….. → cC+ ….
R ∝[A]m[B]n or R = k[A]m[B]n….
Where m and n may or may not be equal to a & b.
m is order of reaction with respect to A and n is the order of reaction with respect to B.
m + n +… is the overall order of the reaction.
Elementary Reaction:
- It is the reaction which completes in a single step.
- A reaction may involve more than one elementary reactions or steps also.
- Overall rate of reaction depends on the slowest elementary step and thus it is known as rate determining step.
Molecularity of Reaction:
- Number of molecules taking part in an elementary step is known as its molecularity.
- Order of an elementary reaction is always equal to its molecularity.
- Elementary reactions with molecularity greater than three are not known because collisions in which more than three particles come together simultaneously are rare.
Chemical Reaction | Molecularity |
PCl5 → PCl3 + Cl2
|
Unimolecular
|
2HI → H2 + I2
|
Bimolecular
|
2SO2 + O2 → 2SO3
|
Trimolecular
|
NO + O3 → NO2 + O2
|
Bimolecular
|
2CO + O2 → 2CO2
|
Trimolecular
|
2FeCl3 + SnCl2 → SnCl2 + 2FeCl2
|
Trimolecular
|
Differential and Integrated Rate Laws:
Zero Order Reactions:
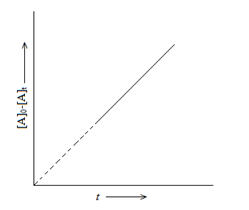
For Reaction: A → Product
[A]0-[A]t = k0t
Where,
[A]0 = Initial concentration of A
[A]t = Concentration of A at time t.
k0 = Rate constant for zero order reaction.
Half Life:
t1/2 = [A]0/2k
Unit of rate constant = mol dm-3s-1
Examples:
- Enzyme catalyzed reactions are zero order with respect to substrate concentration.
- Decomposition of gases on the surface of metallic catalysts like decomposition of HI on gold surface.
First Order Reactions:
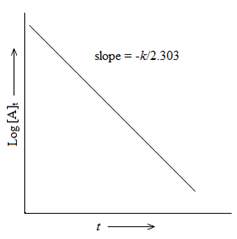
A → Product
(Δ [A] /A) = -k1Δt
or k1=( 2.303/ t)log ([A]0 / [A]t)
Half Life:
t1/2 = 0.693/k1
Half life is independent of the initial concentration of the reactant for a first order reaction.
Units of k1 = s-1
Examples:
N2O5 → 2NO2 + 1/2O2
Br2 → 2Br
2HNO3 → 2NO + H2O
H2O2→ H2O + 1/2O2
Pseudo First Order Reactions:
These are the reactions in which more than one species is involved in the rate determining step but still the order of reaction is one.
Examples:
- Acid hydrolysis of ester: CH3COOEt + H3O+ →CH3COOH + EtOH
- Inversion of cane sugar:
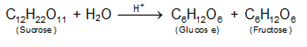
- Decomposition of benzenediazonium halides C6H5N=NCl +H2O → C6H5OH +N2+HCl
Half – Life of a nth Order Reaction:
kt1/2 = (2n-1-1)/(n-1)[A0]n-1
Where, n = order of reaction ≠1
Parallel Reactions:
The reactions in which a substance reacts or decomposes in more than one way are called parallel or side reactions.
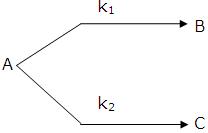
If we assume that both of them are first order, we get.
![-\frac{d[A]}{dt} = (k_1 +k_2) [A] =k_{av}[A]](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latex-_fracdadtk_1k_2ak_ava.jpg)
k1 = fractional yield of B × kav
k2 = fractional yield of C × kav
If k1 > k2 then
A → B main and
A → C is side reaction
Let after a definite interval x mol/litre of B and y mol/litre of C are formed.
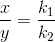
i.e
![\frac{\frac{d[B]}{dt}}{\frac{d[C]}{dt}} =\frac{k_1}{k_2}](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latex_frac_fracdbdt_fracdcdt_frack_1k_2.jpg)
This means that irrespective of how much time is elapsed, the ratio of concentration of B to that of C from the start (assuming no B and C in the beginning ) is a constant equal to k1/k2.
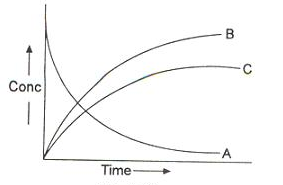

Sequential Reactions:
This reaction is defined as that reaction which proceeds from reactants to final products through one or more intermediate stages. The overall reaction is a result of several successive or consecutive steps.
A → B → C and so on
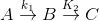
![-\frac{d[A]}{dt} = k_1[A]](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latex-_fracdadtk_1a.jpg) …....(i)
…....(i)![\frac{d[B]}{dt} = k_1[A]-K_2[B]](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latex_fracdbdtk_1a-k_2b.jpg) …......(ii)
…......(ii)![\frac{d[C]}{dt} = k_2[B]](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latex_fracdcdtk_2b.jpg) ….......(iii)
….......(iii)
Integrating equation (i), we get
![[A]-[A]_oe^{-k_1t}](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latexa-a_oe-k_1t.jpg)
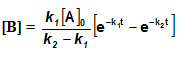
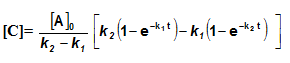
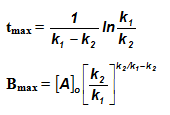
Arrhenius Equation:
k = A exp(-Ea/RT)
Where, k = Rate constant
A = pre-exponential factor
Ea = Activation energy
Temperature Coefficient:
The temperature coefficient of a chemical reaction is defined as the ratio of the specific reaction rates of a reaction at two temperature differing by 10oC.
μ = Temperature coefficient= k(r+10)/kt
Let temperature coefficient of a reaction be ' μ ' when temperature is raised from T1to T2; then the ratio of rate constants or rate may be calculated as
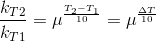
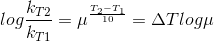
![\frac{k_T_2}{k_T_1}= antilog[\frac{\Delta T}{10 }] log\mu](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latex_frack_t_2k_t_1antilog_frac_deltat10log_mu.jpg)
Its value lies generally between 2 and 3.
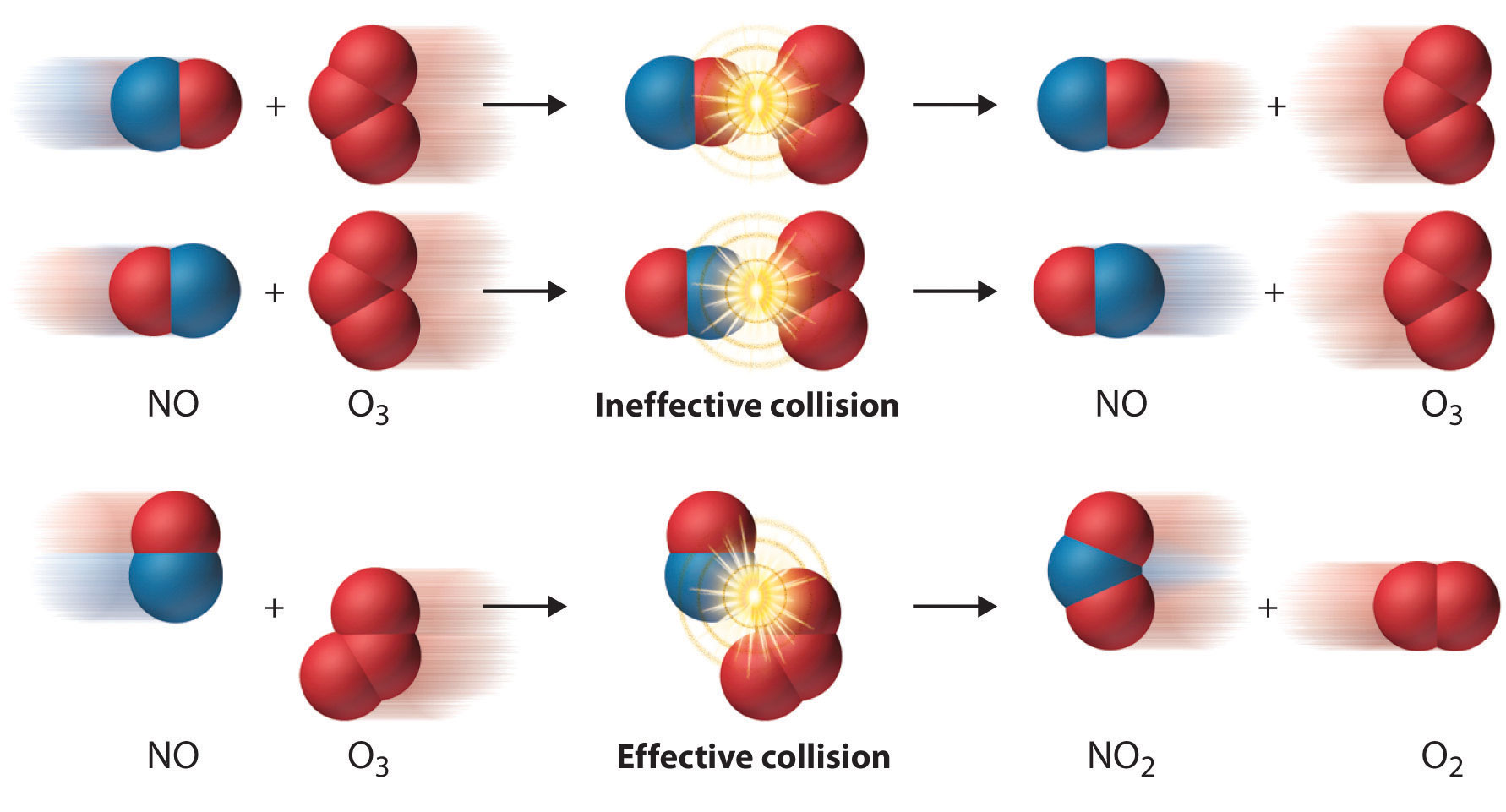
Collision Theory of Reaction Rate
- A chemical reaction takes place due to collision among reactant molecules.
- The number of collisions taking place per second per unit volume of the reaction mixture is known as collision frequency (Z).
- The value of collision frequency is very high, of the order of 1025 to 1028 in case of binary collisions.
- Every collision does not bring a chemical change.
- The collisions that actually produce the products are effective collisions.
- The effective collisions which bring chemical change are few in comparison to the form a product are ineffective elastic collisions, i.e., molecules just collide and
- disperse in different directions with different velocities.
- For a collision to be effective, the following two barriers are to be cleared.
- Energy Barrier
- Orientation Barrier
Radioactivity:
All radioactive decay follow 1st order kinetics
For radioactive decay A ->B
-(dNA/dt) =l NA
Where, l = decay constant of reaction
NA = number of nuclei of the radioactive substance at the time when rate is calculated.
Arrhenius equation is not valid for radioactive decay.
Integrated Rate Law: Nt = Noe-lt
Half Life: t1/2= 0.693/λ
Average life time: Life time of a single isolated nucleus, tav= 1/λ
Activity: Rate of decay
A = dNA/dt, Also, At = Aoe-lt
Specific Activity: activity per unit mass of the sample.
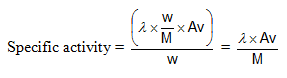
Units: dps or Becquerrel

0 comments:
Post a Comment