Drugs:
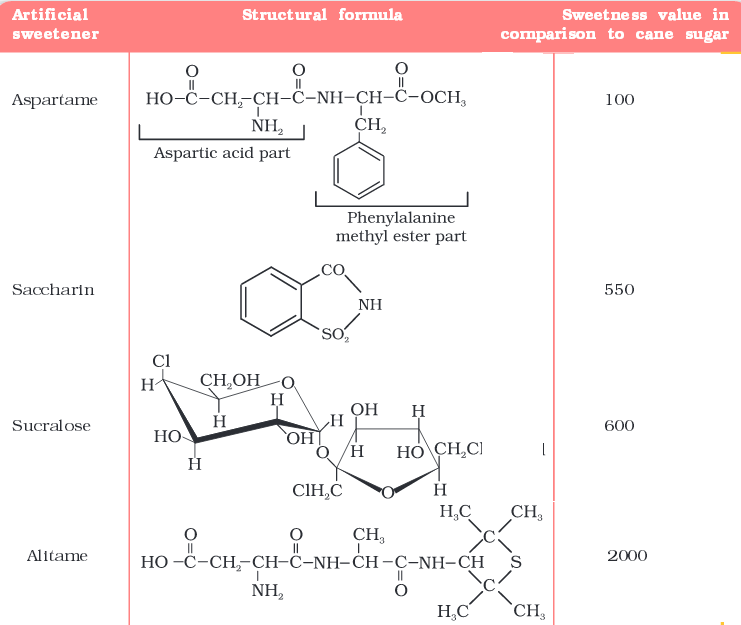
Artificial Sweetening Agents
Food preservatives:
These are the chemical substances which prevent undesirable changes in flavor, colour, texture of the food during processing and storage of food.Examples, Table salt, sugar, vegetable oils, sodium benzoate (C6H5COONa) etcCleansing Agents
Soaps:Sodium or potassium salts of fatty acids.Soaps do not work with hard water as it forms insoluble salts with calcium and magnesium ions present in hard water.Detergents:Sodium or potassium salts of sulphonic acids. These can work with hard water also.Anionic Detergents: Sodium Slats of sulphonated long chain alcohols or hydrocarbonsCationic Detergents: Quaternary ammonium salts of ammines with acetates, chlorates or bromates.Non-Ionic Detergents: Do not contain any ion.
Sunday, May 27, 2018
Subscribe to:
Post Comments (Atom)



0 comments:
Post a Comment